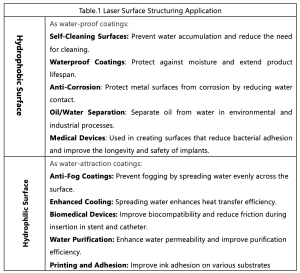
Both hydrophobic and hydrophilic surfaces have a broad range of practical industry applications due to their unique interactions with water. Table 1 shows some key application areas for each.
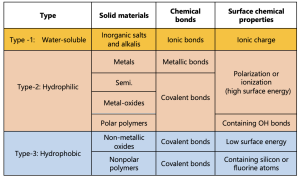
Hydrophobic and hydrophilic surfaces naturally occur in nature, and humans have long sought to replicate these properties on various material surfaces. Through extensive studies, researchers aim to mimic these natural behaviors while gaining a deeper understanding of the underlying physics and chemistry. A material’s hydrophobic or hydrophilic behavior is primarily determined by the surface’s chemical polarity, which is closely related to the molecular structure of water (H₂O). The O-H bond in the water molecule (H-O-H) is highly polar, making water a polar molecule. When water interacts with solid materials, several outcomes can occur: (i) dissolution of the solid (common with most inorganic salts and alkalis), (ii) water adsorption without dissolution (typical for metals, semiconductors, oxides, and some polymers), or (iii) rejection of water through physical interactions (observed in certain polymers). Table 1. categorizes solid materials based on their inherent characteristics, distinctly classifying them as water-soluble, hydrophilic, or hydrophobic.
In this article, we will focus on type2 and type-3 of material surface interaction with water. There are established standards for defining hydrophobic characteristics, primarily based on the contact angle between water droplets and the material surface as shown in Figure 1. The larger the contact angle, the stronger the hydrophobic performance.
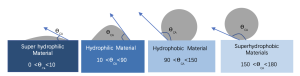
Figure 1. Schematic Diagram of the Relationship between Hydrophilic and Hydrophobic Characteristics and Contact Angles
To meet the requirements of device applications, certain intrinsically hydrophilic functional materials must modify their surface properties to become hydrophobic, or vice versa. Currently, two primary techniques are commonly employed: (i) applying a hydrophobic polymer with Si-or F-group or hydrophilic polymers with OH- or COOH-group directly to the surface, and (ii) fabricating micro- or nanostructures directly onto the surface.
External polymer coatings can limit certain critical applications, especially in the case of biomedical devices where contamination from foreign materials needs to be prevented. Laser technology offers unparalleled precision and control, making it uniquely suited for fabricating hydrophobic and hydrophilic surfaces. Lasers can achieve these modifications without the need for additional chemicals, allowing for clean, environmentally friendly processing. Moreover, the versatility of laser systems enables the tailoring of surface properties on a wide range of materials, making them ideal for applications across various industries, including biomedical devices, electronics, and coatings. Especially, with the “cool processing” trick of femtosecond lasers, designed structures can be realized. The industrialization of femtosecond lasers has brought the rapid development of hydrophobic and hydrophilic surface preparation technology closer to achieving industrial goals.
Although there are quite broad range of applications of hydrophobic and hydrophilic surface functionality, laser fabrication is purposely employed for certain high-ends device, especially medical devices, requiring super-hydrophobic and hydrophilic surface, such as metal surface anti-corrosion, semiconductor surface anti-wetting, and polymer surface enhancing wetting etc.
The ideal laser sources for surface structuring are fs lasers, which could maximumly reduce thermal effect as shown in Figure 2.
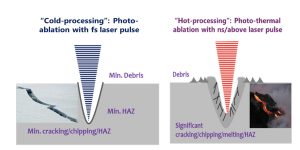
Figure 2. Comparison between laser cold processing and thermal processing
AOC offers the industry a reliable, around-the-clock dural-wavelength fs laser system with wavelengths of 1030 nm (IR) and 515 nm (GR), ideal for surface structuring applications. Figure 3 provides the specifications for this dual-wavelength fs laser.

Figure 3. AOFemto Jericho Series Industrial Green (515nm)/IR (1030nm) femtosecond laser.
Since the development of its first fs laser, AOC has made significant contributions to fs laser surface structuring across a variety of materials, including metals, semiconductors, glass, and polymers. Our applications include marking, polishing, tribology, enhancing adhesion, and modifying hydrophobic/hydrophilic properties.
In this section, we will focus on our advancements in using fs laser technology to create hydrophobic surfaces on metals and hydrophilic surfaces on polymers.
Figure 4 illustrates the surface micro/nano-structuring achieved using fs-GR laser processing on various materials, including stainless steel (SUS), copper (Cu), nickel (Ni)/alloys, titanium (Ti)/alloys, NiTi, and silicon (Si), all under identical processing conditions. While all samples exhibit clear nanostructures superimposed on the micro-structured surface created via direct laser writing, the resulting surface structures vary slightly due to the differing material properties of each substrate.

Figure 4. Femtosecond Laser Fabrication of Micro-Nano Structures on Various Surfaces
The hydrophobic behavior test was conducted on laser-structured metal and silicon surfaces, with the samples left in air for 2 days for Cu and 10 days for SUS after laser structuring. Interestingly, only the structured Cu and SUS surfaces exhibited hydrophobic properties, likely due to differences in their chemical characteristics. Figure 5 shows the water droplets on the structured Cu and SUS surfaces, with contact angles of 144.4° on Cu and 116° on SUS, respectively.

Figure 5: Analysis of hydrophobic performance of fs-laser structured Cu and SUS surface
Another typical example is the use of femtosecond (fs) laser structuring of polymethyl methacrylate (PMMA) to enhance hydrophilic properties, which is particularly beneficial for polymer-based microfluidic devices. A similar laser structuring process with lower pulse energy was utilized. PMMA is unique in nature, exhibiting a contact angle (CA) of 70° due to the presence of both non-polar (-CH3) and polar (-COOCH2) groups. As a result, its surface can be modified to become either highly hydrophobic or hydrophilic using appropriate surface treatments such as laser modification, chemical processing, or plasma treatments. In this article, we will introduce AOC’s approach of enhancing surface hydrophilic performance by delicately tuning laser parameters.
Figure 6 shows the water droplets on the structured PMMA surfaces, demonstrating a gradual reduction in contact angles, from 70° on the untreated surface to 58°, 50°, 46°, 42°, 39°, and 37° on the laser-structured surfaces. These variations were precisely achieved by optimizing key laser parameters.
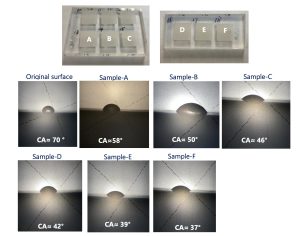
Figure 5: Analysis of hydrophilic performance of fs-laser structured PMMA surface with tuning core laser parameters.
In summary, this effort is ongoing. AOC’s strategic plan focuses on developing laser micro-structuring processes to create tailored hydrophobic and hydrophilic surfaces on a range of functional materials, enabling various applications.